- Zantac Lawsuit Guide – The Link Between Zantac & Cancer - November 3, 2021
- Uber Lawsuit Guide: All You Need To Know - October 20, 2021
- Lipitor Lawsuit Explained: The Link Between Lipitor & Diabetes - September 20, 2021
Valsartan is a blood pressure drug that trades under the brand name Diovan. In 2018, the FDA announced a series of voluntary recalls by manufacturers of valsartan due to the presence of certain impurities in a number of batches of the medication.
These impurities were carcinogenic, which means that they may have increased the risk of users developing cancer. While manufacturers have cleared up this issue since its discovery, many valsartan patients may have been exposed to severe cancer risk while these impurities were present.
If you were taking valsartan in the period leading up to its recall, you may be entitled to compensation from the manufacturers.
If this is your situation, you need to learn more about valsartan lawsuits and whether you might be entitled to take a case.
What Is Valsartan?
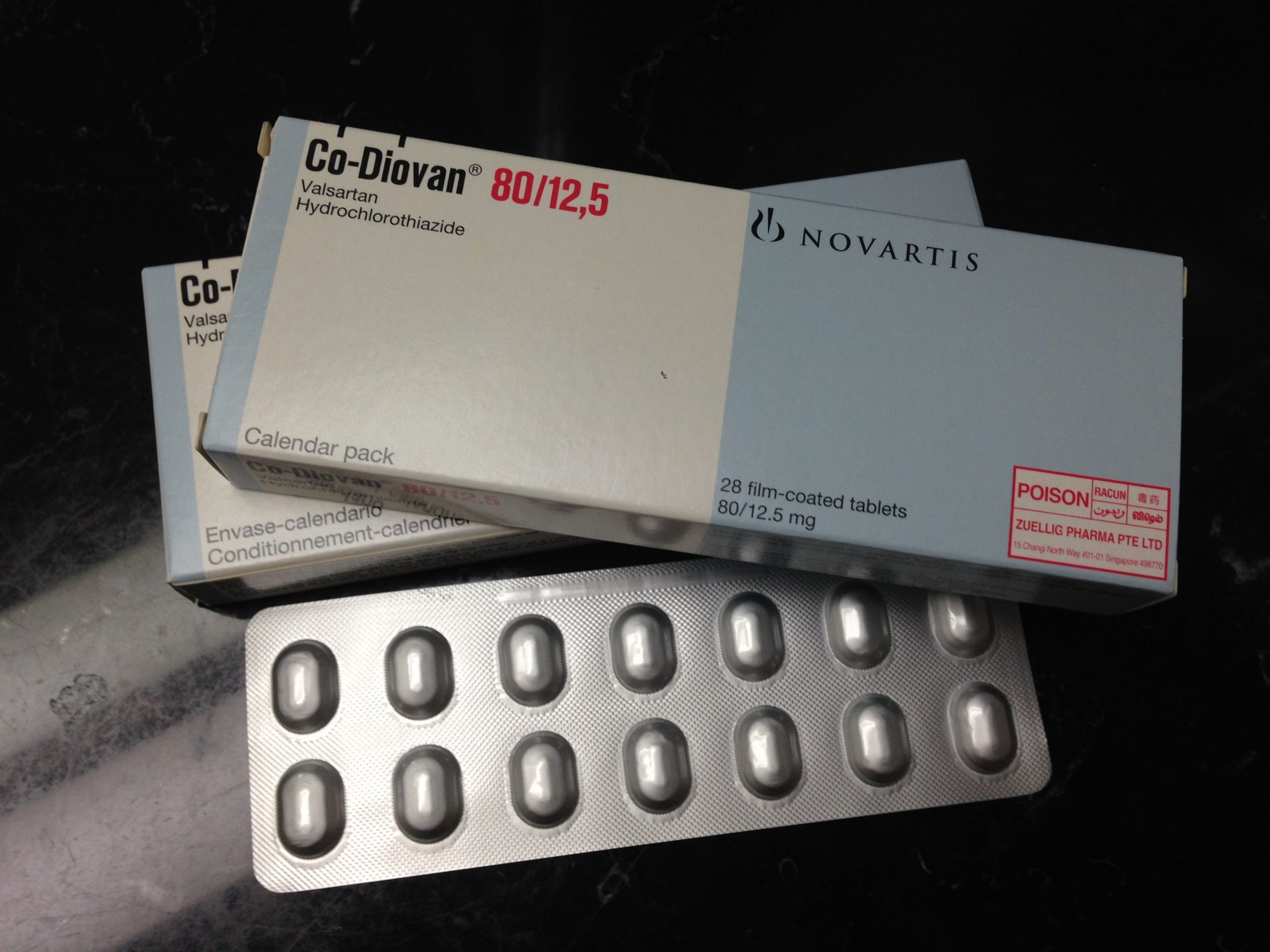
Valsartan is the active pharmaceutical ingredient (API) of a number of blood pressure medications. Novartis originally released the medication under the brand name Diovan; this product is still on pharmacy shelves today.
However, once valsartan’s patent ran out, other drug companies began to make generic versions of Diovan. Many different pharmaceutical products (and companies) were therefore affected by these issues and the resulting lawsuits.
Under normal circumstances, valsartan treats high blood pressure by blocking angiotensin receptors. This allows blood to travel around the body more easily. Patients take valsartan orally, either as a tablet or a liquid solution. Diovan and its generic substitutes are prescription medications. Only patients whose doctors direct them to take valsartan can get it.
The Key Legal Issue
Manufacturers and suppliers of any product have a legal obligation to make that product safe for consumers. Pharma companies failed to fulfill that obligation by providing contaminated medicine to their customers.
Because Valsartan is an essential prescription medication, this failure is even more problematic. A patient who takes Valsartan does so because they are directed to take it by their doctor. Failure to take it could result in serious health issues.
This left many patients in a position where they had to continue taking valsartan despite knowing of the contamination risk. Because these pharma companies breached the duty of care that they owed to their customers under civil law, their customers are entitled to sue them for damages.
The Recall of Valsartan
Firstly, it is important to note that there was never an official FDA recall of any valsartan product. While the FDA published details of the manufacturing issues and warned consumers and retailers to be vigilant, they never took steps to remove valsartan products from the market.
However, many manufacturers did recall their valsartan products voluntarily. As noted above, valsartan was not protected by a patent after 2012, which meant that many pharmaceutical companies were making it.
Why Was Valsartan Recalled?
The safety issues with valsartan revolve around impurities that ended up in the medications during the manufacturing process. The contaminants in question were probable or potential carcinogenic compounds.
NDMA, NDEA, and NMBA may all cause various types of cancer in humans. Analysts found these compounds in various quantities in different valsartan products.
These impurities began appearing in valsartan after a new manufacturer in China began to produce pills for a number of companies. They engaged in the negligent practice of reusing solvents to cut costs, which led to the contamination.
At the time of the contamination, this manufacturer produced the majority of the world’s valsartan.
Recalls & Warnings
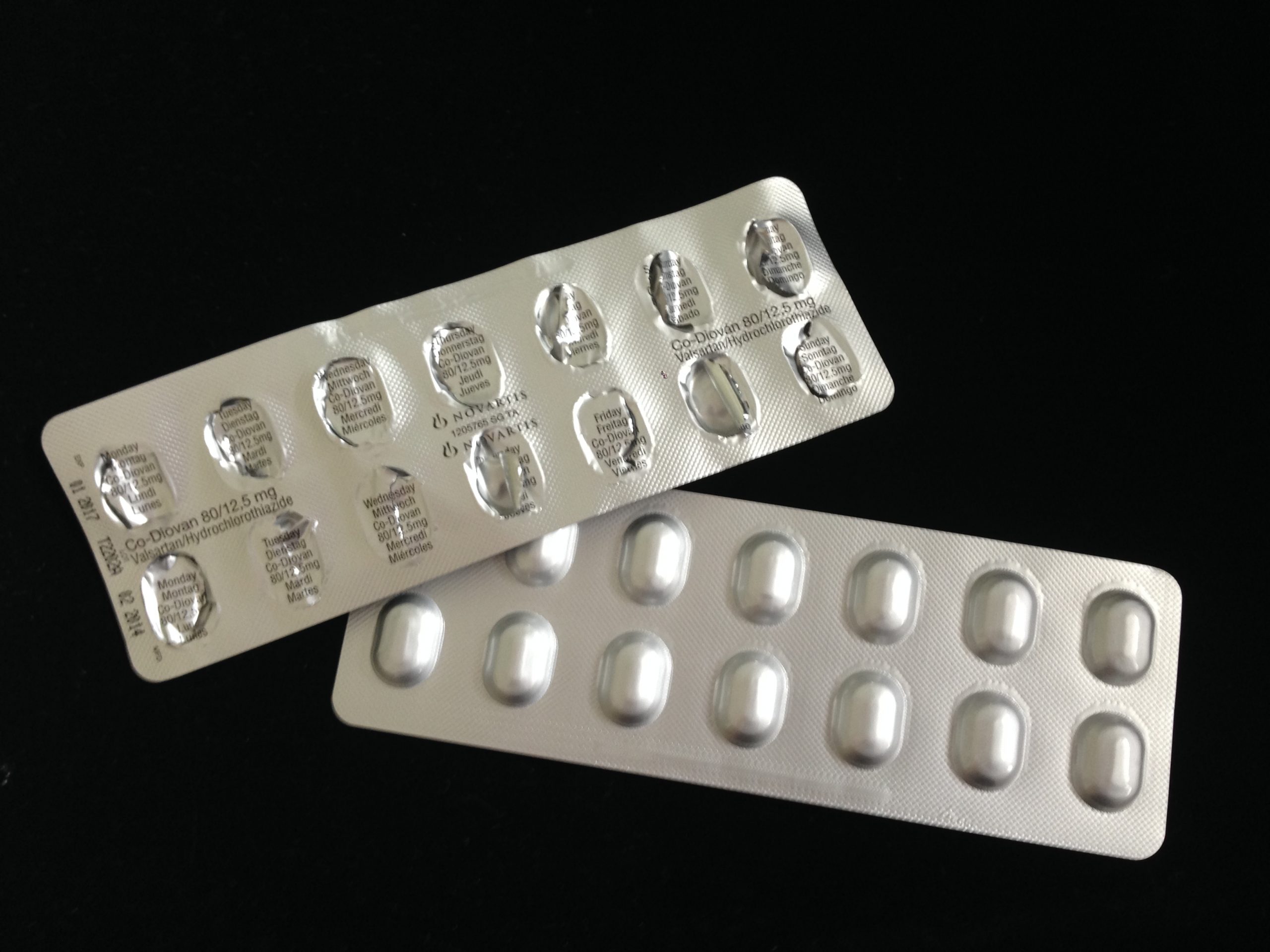
As noted above, there are many different Valsartan products available in pharmacies. While many of these were affected by contamination issues, not all were.
Also, not every company took the same approach in terms of recalls. Some chose to recall all products immediately, while others issued partial recalls or warnings. The first recalls took place in July 2018. Since then, more than 15 companies have chosen to pull their valsartan products from store shelves.
The companies that recalled their products are:
- A-S Medication Solutions
- Acetris Health
- Actavis Pharma
- American Health Packaging
- Aurobindo Pharma USA
- AvKare
- Bryant Ranch Prepack
- Camber Pharmaceuticals
- H J Harkins Company
- Major Pharmaceuticals
- Mylan Pharmaceuticals
- Northwind Pharmaceuticals
- NuCare Pharmaceuticals
- RemedyRepack
- Solco Healthcare
- Teva Pharmaceuticals
- Torrent Pharmaceuticals
The FDA is the body in charge of maintaining this list and adding to it as pharma companies release updates or announce new recalls.
Medications containing losartan and irbesartan, which are similar treatments for high blood pressure, were also recalled due to contamination. New information on this case becomes available all the time. You can check the FDA website to find out the status of your specific medication.
What Is the Legal Basis for a Lawsuit Against Valsartan?
In order for you to have a case against valsartan, you need to have been taking one of the affected medications for at least a year between 2014 and 2018. You also need to have developed one of the cancers that are listed as risks.
These cancers include:
- Liver cancer
- Intestinal cancer
- Pancreatic cancer
- Non-Hodgkins Lymphoma
- Leukemia
- Esophageal cancer
- Prostate cancer
- Multiple myeloma
When building a Valsartan lawsuit, there are a couple of different approaches open to lawyers. In order for liability to attach to a defendant in a civil case, the plaintiff’s team will have to prove to a judge that the defendant committed a specific wrong. In the case of Valsartan, there are two main wrongs that may apply.
Failure to Warn
Valsartan is a potentially dangerous medication due to possible side effects. Manufacturers have a duty to warn patients of these side effects with appropriate labeling. Valsartan manufacturers do list many side effects, thereby discharging their duty to warn in relation to those risks. However, no company warned consumers about the possibility their medication might contain carcinogens.
To succeed here, you would have to show that you would not have consumed a valsartan product had the medication’s packaging informed you of this possibility.
Defective Product
This is the head of liability under which most valsartan lawsuits will come. Because the medications contained harmful impurities, they are legally considered to be defective products.
If you pay for a defective product which harms your health, you have a legal right to compensation from the manufacturer. All you have to prove is that you bought a defective product and that this product caused you to become ill (in this case, with cancer).
A potential issue here is that of causation. Many different things cause cancer, so you may have difficulty proving that the medication was at fault in your case.
What Should I Do If I Have Been Affected by These Issues?

If you were taking valsartan at a time when it could have contained impurities, and you later received a cancer diagnosis, you may be entitled to compensation from the manufacturer of the specific product you took. However, you won’t get this automatically.
To pursue your entitlements, you’ll have to file a lawsuit. The first step here is hiring a lawyer.
Hiring a Lawyer
When selecting an attorney to work with on a Valsartan lawsuit, there are a couple of important factors to keep in mind. Firstly, you want someone with experience in this area of civil law. You should look for an attorney with experience in defective product cases, and preferably a good record in terms of settlements.
Additionally, it’s a good idea to hire someone that understands your case on a personal level. You may end up dealing with this person extensively, so you need someone who is sensitive to what you’ve gone through and can offer emotional support if the case becomes distressing.
Building a Case
Once you’ve got legal counsel on your side, it’s time to start building your case. You’ll have to compile detailed records of your history with Valsartan.
Ask your doctor for the medical records that show when you began taking the medication. Also, make sure to keep any pill bottles you might have from the period when contamination may have been an issue. Not every Valsartan brand was affected by the contamination. You’ll have to check that the brand you used had impurities before pursuing your case.
You may never have to actually go to court to seek compensation. Once the initial Valsartan lawsuits reach a conclusion, the level of compensation that plaintiffs are likely to receive will become clear. At that point, you might well be able to negotiate a settlement in private, without the involvement of the court.
What Would I Get From a Successful Valsartan Lawsuit?
The monies you receive when you win a civil case are called damages. The amount of these damages will depend on a few different factors.
Firstly, any medical expenses you incur due to a valsartan-related illness should be covered. This includes hospital stays, assessments by doctors, and ongoing outpatient care. If you were forced to miss work due to a Valsartan-related illness, you should also receive lost wage replacement as part of your settlement. This will not always replace your entire wage, but it should make up most of it.
In a case like this, pain and suffering damages may also be significant, depending on the extent of the harm you suffered. These damages are a means of compensating plaintiffs for difficulties they have experienced with a monetary payment.
Aside from these main damages, you may also receive compensation for any miscellaneous expenses you had to cover during the course of your difficulties with valsartan. Transport costs you incurred while going to and from medical appointments would be an example of this.
Lump-Sum or Structured Settlement?
Because of the likely size of a valsartan settlement, as well as your ongoing requirements in terms of medical bills and wage replacement, a judge might choose to award your compensation in the form of a structured settlement. This would mean that you’d receive regular payments over a long period, rather than a single lump sum.
In order to avoid payment issues, courts usually decree that an escrow account holder takes charge of structured settlement funds. This means that the defendant would be unable to interfere with your payments.
How Long Would Settlement Money From a Valsartan Lawsuit Take to Arrive?
As you’ll know if you’ve ever been through the courts before, legal issues like this can take a long time to reach a conclusion. Any settlement money you end up getting is unlikely to be in your account any less than a couple of years after you first decide to take a case.
At the moment, the first Valsartan lawsuits are still underway. When the courts hearing these cases come to verdicts, it will pave the way for future Valsartan suits.
The time it takes to settle these cases decreases for every new settlement reached. Once plaintiffs and defendants come to understand roughly how much compensation is appropriate in different scenarios, settlements will not take as long to negotiate.
Can I Sue as Part of a Class Action Lawsuit?
A class-action lawsuit is a case where multiple plaintiffs sue the same defendant for the same reason. The class action structure can help individual plaintiffs to limit legal costs and minimize their personal involvement with the case.
The valsartan lawsuits currently underway have adopted multi-district litigation (MDL) structure. This is similar to a class action in that several plaintiffs come together to sue one defendant.
With an MDL, however, each plaintiff takes their own individual case. Under a class action structure, all cases are combined into one. Going forward, those affected by Valsartan issues may choose to combine their legal efforts under a class action. This would be more suitable for plaintiffs whose illnesses were relatively minor.
Securing Your Entitlements With a Valsartan Lawsuit
When you rely on medication to keep you healthy, the last thing you expect is for that medication to make you ill. Unfortunately, this can and does happen.
If your health has suffered due to faulty medication, whether it’s valsartan or another type of medicine, you have the right to take legal action. This is the only way to secure justice for the harm you have been exposed to. To learn more about what’s involved in a valsartan lawsuit, contact us today.
FAQs About Valsartan
How Dangerous Are the Contaminants?
There are three suspected carcinogenic chemicals that have emerged in valsartan; NDMA, NDEA, and NMBA.
Of these, NDMA is the most problematic. NDMA has the classification of “probable human carcinogen.” Researchers have been able to confirm that it causes cancer in animals.
NDEA is also a probable human carcinogen. While it is not present in valsartan in the same quantities as NDMA, it also poses a serious threat.
Is It OK to Simply Stop Taking Valsartan?
This is one of the more difficult issues in the discussion around Valsartan impurities. While no doctor wants to advise their patient to take a medication that potentially has carcinogenic impurities in it, they cannot instruct anyone to stop taking a potentially life-saving treatment without introducing an alternative.
The FDA has determined, however, that the real risk of illness developing from taking Valsartan is low. They estimate that the impurities would have resulted in one cancer diagnosis for every 8,000 patients. Because of the much higher risk associated with quitting an essential medicine, the official advice is to keep taking Valsartan unless your doctor recommends coming off it.
What Are the Ordinary Side Effects of Valsartan?
As noted above, valsartan medications come with a range of side effects under normal circumstances.
These are a result of the medication as it is marketed; the presence or absence of impurities has no bearing on them.
You will only be able to sue a company in respect of one of these side effects if the packaging of their product fails to warn of it.
Minor valsartan side effects include:
– Dizziness
– Nausea
– Diarrhea
– Fatigue
– Pain in the back or joints
More serious potential side effects of valsartan include:
– Kidney disease
– Fainting from low blood pressure
Valsartan is unsafe for pregnant women, or women planning to become pregnant in the near future. It could seriously damage or terminate your fetus if you take it during pregnancy.
Is It Safe to Take Valsartan Now?
If you have recently received a diagnosis of high blood pressure, you may be wondering whether it’s safe for you to begin a course of treatment involving valsartan. Unfortunately, many of the product recalls we’ve listed here are still ongoing.
However, a new generic valsartan medication was FDA-approved early last year. The FDA carried out an inspection of the manufacturing facility in India before granting approval for the medication. There are also a number of valsartan products that did not have to be recalled.
Therefore, you should not be concerned if you need to begin taking valsartan in 2020.
Works Cited
- Food and Drug Administration, (July 13, 2018). FDA announces voluntary recall of several medicines containing valsartan following detection of an impurity. Retrieved from: https://www.fda.gov/news-events/press-announcements/fda-announces-voluntary-recall-several-medicines-containing-valsartan-following-detection-impurity
- Mayo Clinic, (August 16, 2019) Angiotensin II receptor blockers. Retrieved from: https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/in-depth/angiotensin-ii-receptor-blockers/art-20045009
- Food & Drug Administration, (n.d.) Search List of Recalled Angiotensin II Receptor Blockers (ARBs) including Valsartan, Losartan and Irbesartan. Retrieved from: https://www.fda.gov/drugs/drug-safety-and-availability/search-list-recalled-angiotensin-ii-receptor-blockers-arbs-including-valsartan-losartan-and
- Stanford Encyclopedia of Philosophy, (October 3, 2019) Causation in the Law. Retrieved from: https://plato.stanford.edu/entries/causation-law/
- Investopedia (May 17, 2019) Escrow. Retrieved from: https://www.investopedia.com/terms/e/escrow.asp
- The Balance Small Business (July 21, 2020) What Is a Class Action Lawsuit? Retrieved from: https://www.thebalancesmb.com/what-is-a-class-action-lawsuit-3623787
- Food and Drug Administration (March 12, 2019) FDA approves a new generic valsartan. Retrieved from: https://www.fda.gov/news-events/press-announcements/fda-approves-new-generic-valsartan
